Introduction
The benefits of natural supplementation in medicine has stimulated the development of functional and integrative dermatology.1 Inositol (Ins) is a family of naturally occurring cyclohexanehexol (myo-, scyllo-, muco-, neo-, and D-chiro-Inositol) and its derivatives (L-chiro-, allo-, epi-, cis-Inositol) that has received much interest.2 Myo-inositol (MI) and D-chiro-Inositol (DCI) have been shown to improve insulin resistance and decrease total testosterone, granting their utility in patients with polycystic ovarian syndrome (PCOS) and hyperandrogenism.3–5
Over the past years, MI was used for the treatment of metabolic syndrome, reproductive diseases, and pregnancy development. Given the intricate link of skin health to hormones and metabolism,6 this systematic review will explore the potential roles of Ins in skin diseases, including acne vulgaris, hirsutism, psoriasis, seborrheic dermatitis (SD), hidradenitis suppurativa (HS), trichotillomania, and melanoma.
Materials and Methods
Literature Search
This study was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).7 A primary literature search was conducted with MEDLINE, EMBASE, and Cochrane Library databases on December 20, 2021, with no limitation on date. The search terms included: (“Acne Vulgaris” OR “Acne” OR “Dermatitis” OR “Skin Diseases” OR “Skin” OR “Skin Physiological Phenomena” OR “Skin Abnormalities” OR “Dermatology” OR “Cutaneous”) AND (“Myo-inositol” OR Inositol). The full list of the search terms is available in online supplementary materials (Table S1).
Study selection and appraisal
Two reviewers (T.L and Y.C) independently screened all articles to include clinical trials and case series written in English or Chinese on Ins and skin disorders. Any discrepancies were resolved with a third reviewer (F.C). Animal studies, reviews, and those not discussing skin diseases were excluded. Additional studies meeting the criteria were identified from references and included if the above-mentioned criteria were met.
Data extraction and quality assessment
Quality of evidence was determined per the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence.8 The Cochrane Risk of Bias tool was used for randomized controlled trials (RCTs),9 the Risk of Bias in Non-randomized Studies of Interventions was used for non-RCTs,10 and the National Institute of Health quality assessment tool was used for case reports and case series.11
Results
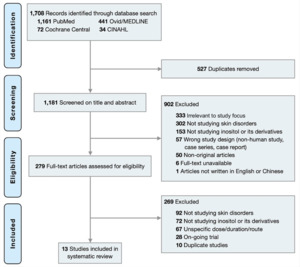
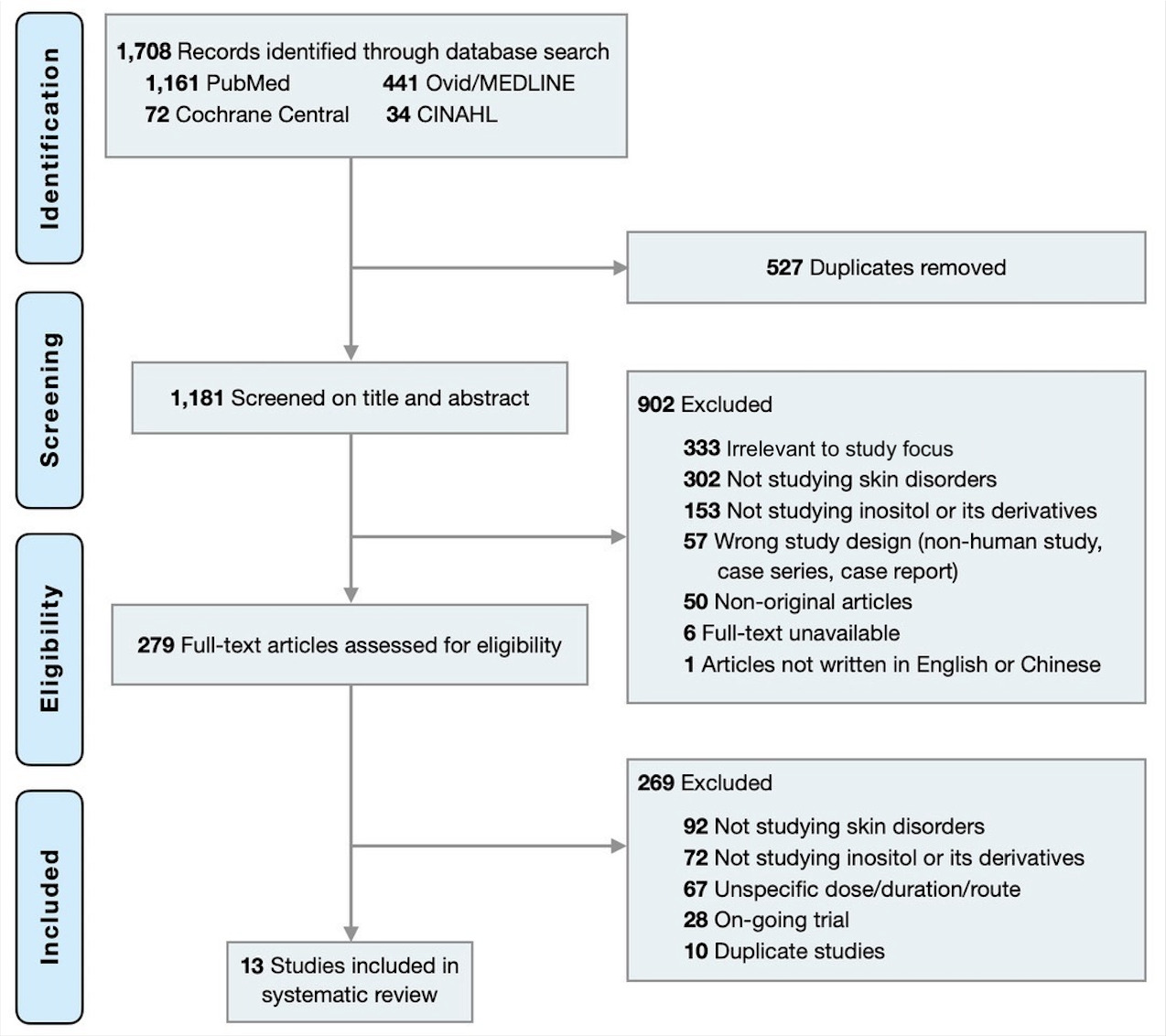
The initial search identified 1,181 records non-duplicate articles from the database. A total of 282 studies met the inclusion criteria. After full-text review, 13 studies including 6 randomized controlled trials (RCTs),12–17 five non-RCTs,18–22 one case series,23 and one case report24 were included in this systematic review (Figure 1).
Hyperandrogenism: acne and hirsutism
Acne
Oral Ins as a treatment for acne has been described in four clinical trials (total n = 204) and one study using topical MI (n = 40). In a study by Fruzzetti et al, 38% of PCOS females who consumed 4 g MI plus 400 mcg folic acid daily reported improvement in acne symptoms.12 Although these PCOS women had a higher average testosterone level, Ins did not consistently affect patients’ androgen levels. Advani et al assessed the effect of twice daily oral combined 600 mg Ins (MI+DCI) on 51 PCOS women with normal (≤23) or high (>23) body mass index (BMI).18 They revealed a larger decrease in acne scores in overweight or obese subjects compared to lean individuals (-10.05 vs -4.38), although both groups achieved significant acne clearance within a 12-week treatment course. Another study assessing 2 g Ins twice daily in 50 PCOS women found decreased numbers of papulopustular lesions.13 Ramanan et al conducted a trial on 32 females with mild-to-moderate acne and hirsutism but no comorbid endocrine and metabolic diseases.20 Their result revealed a significant reduction of the modified Cook’s acne scale from 4.34±0.33 to 1.3±0.17 after 24 weeks of twice daily 2 g MI. Lastly, Fabbrocini et al tested topical Ins on 40 women with adult female acne.22 Participants were instructed to apply 4% MI peel-off mask every other night for 2 months. The study reported a 45-69% reduction in mean acne counts.22
Hirsutism
We identified three clinical trials on hirsute women with or without PCOS using oral Ins (n = 129). Minozzi et al prescribed 2 g MI twice daily for 6 months to 46 women with mild to moderate hirsutism and found a significant decrease in hirsutism scores (-2.3±0.9, p < 0.001) and total androgens (-13±2.6, p < 0.002) from baseline.21 Another study by Ramanan et al followed 32 women with the same inclusion criteria and intervention and found a time-dependent reduction in modified Ferriman-Gallwey hirsutism score from 10 to 5.8.20 Additionally, 60% fewer patients were complaining of hair loss at the end of the study. The other study by Advani et at reported a reduction in hirsutism score of (-0.45, p < 0.01) in obese PCOS women and (-0.25, p < 0.05) in lean PCOS women.18
Inflammatory dermatoses: psoriasis, seborrheic dermatitis, and hidradenitis suppurativa
Psoriasis
The use of Ins in psoriasis has been reported in two randomized controlled trials (n = 69). Allan et al published a study on 23 patients with concomitant plaque psoriasis and bipolar disorder using the oral preparation of 6 g Ins daily for 2.5 months.16 Improvement of psoriasis, as measured by Psoriasis Area and Severity Index score (PASI), was seen in the subgroup of patients taking lithium but not in the other non-lithium treated subgroup. There was no difference in treatment of bipolar disorder appreciated in patients with use of Ins. Another study by Owczarczyk-Saczonek et al investigated topical 0.25% and 1% DCI for the treatment of chronic plaque psoriasis on 46 patients and showed subjective and clinical improvement of psoriasis as measured with PASI.14 In addition, the high-dose group showed improvement objective measurement of transepidermal water loss while patients in the low-dose group achieved better clinical outcomes seen in PASI improvement.
Seborrheic dermatitis
A topical gel containing glycerol-phospho-Inositol (GPI) was tested on 25 patients with mild-to-moderate facial SD in an uncontrolled clinical trial.19 They demonstrated a significant reduction in the investigator’s global assessment scale and an excellent response with more than 80% improvement reported in nearly 50% of the cases. However, it was unclear whether the effect was due to GPI or other ingredients in the product. More studies are required to further look at topical inositol as a potential treatment for seborrheic dermatitis.
Hidradenitis suppurativa
Donnarumma et al evaluated the effectiveness of additional oral MI (2 g daily) in 20 hidradenitis suppurativa (HS) patients taking concurrent antimicrobials.15 The study reported a significant reduction of the Sartorious Score in the dual therapy group from 38.3±7.75 to 27.3±8.02 (p < 0.04). On the contrary, the antibiotic monotherapy group improved from 38.4±7.88 to 31.1±8.02 (p = 0.55). This was the only study available looking at inositol as an adjunctive treatment for the treatment of hidradenitis suppurativa.
Trichotillomania
Two studies using Ins in patients with psychodermatoses (n = 22) were identified. Seedat et al, reported successful treatment of refractory trichotillomania and compulsive skin picking with Ins in 3 cases using daily dose of 18 g/d administered in white powder form.23 The other study was a randomized controlled trial assessing 19 patients with trichotillomania.17 In this study, 42.1% of the 19 patients reported “much or very much improved” with oral Ins 6 to 18 g/day, while 35.3% of the 19 patients on placebo reporting clinical improvement. These studies did not show statistically significant effects of Ins therapy (p > 0.05). Further studies are required to conclude the potential use comorbid psychiatric disorder.
Neoplasm – melanoma
Khurana et al reported a stage IV melanoma patient who had preferred treatment with inositol hexaphosphate (IP6) plus MI (800 mg/220 mg) over standard of care treatment.24 The patient received a total dose of 8 g IP6 and 2.2 g Ins daily for 2 years and when complete, clinical and radiological remission was achieved and no relevant side effects reported. However, this is the only case report reported in the literature.
Discussion
Inositol is a polyol widely distributed in animal and plant cells as phosphorylated derivates and in cell membranes and serves as a fundamental component for cellular membranes and intracellular messengers.16 Inositol’s function in cellular signaling transduction is postulated to be insulin-like in quality,22 and comprising a major role in cellular growth and survival, metabolic regulation, nerve functionality, and reproductive activity.5 Due to its ubiquitous nature, the average human consumes one gram per day of Ins. It also is available without prescription as an oral nutritional supplement but can also be used in topical forms.3,16
Through this systematic review, we have provided quantitative estimates of the efficacy of Ins as adjunctive treatment for various dermatologic pathologies with inflammatory, psychiatric, and neoplastic etiologies. The wide breadth of pathologies highlighted in this review suggest the diverse roles that inositol possibly plays as a cell signal transducer.
Inositol is known to have positive systemic effects consistently found on reproductive disturbance and insulin resistance in populations with PCOS.5 This androgen-lowering property of Ins was investigated as a possible adjunct to acne and hirsutism topical therapy, as acne arises from the interplay between sebaceous glands, skin flora, and androgenic hormones.25 Like most current regimens, effect of Ins on acne may involve multiple mechanisms other than hormonal regulation, including anti-inflammatory effects.26,27 Hirsutism is characterized by excessive male-pattern hair growth in women. Like acne, hirsutism can also be hormonal or idiopathic.25 Ins may improve PCOS-related hirsutism in the same fashion as PCOS-related acne. Two out of three studies investigated hirsutism in non-PCOS women. Thus, synergistic hormonal balance and inflammatory cytokine down-regulation could have contributed to the improvement in either type of hirsutism. Overall, topical Ins may be considered as an adjunctive treatment in acne and hirsute women with PCOS or non-PCOS-related due to its promising reported efficacy and safety profile.
Psoriasis is characterized partly by an overproduction of TNF-α, a key inflammatory cytokine underlying the formation of skin and joint lesions.28 Studies have reported some anti-inflammatory and antioxidant properties of MI and DCI.27 In particular, TNF-α was found significantly suppressed by pinitol, a methylated form of DCI.26 Despite these plausible mechanisms, the role of Ins in psoriasis requires further elucidation. Different responses between the two groups in the study reported by Allan et al’s16 the authors suggested that endogenous Ins, which is inhibited by lithium, may be a sufficient but not necessary molecule for psoriasis pathogenesis.29 Therefore, the efficacy of Ins may depend on patient characteristics and route of administration, more research is warranted to identify possible underlying mechanism.
SD is a common multifactorial inflammatory skin condition associated with aberrant hormone levels, skin flora composition, fatty acid metabolism, and neurogenic factors.30 Recently, Malasseszia was reported to trigger a Type 17 response mediated by IL-17 and IL-23 that exacerbates cutaneous inflammation.31 Future studies should look at the potential role of inositol and immunity against fungal related cutaneous disorders.
HS is a relapsing-remitting inflammatory dermatosis characterized by painful nodules, abscesses, and sinus tract formation.32 Interactions between innate immunity and skin microbiota, and genetic factors have all been linked to the development of HS.33 The inflammatory cytokines at play are very similar to psoriasis, including TNF-α, IL-17, and IL-23.34 Therefore, a detailed understanding of its pharmacodynamics may shed light on the possible roles of Ins in treating both conditions.
MI regenerates hydrolyzed phosphoinositides (PI), the first messenger in multiple neurotransmitter pathways, making it an emerging therapeutic treatment for psychiatric disorders.35 Trichotillomania, characterized by repetitive hair pulling leading to hair loss, is not uncommonly seen in this population. Although selective serotonin reuptake inhibitors were proposed to treat comorbid skin diseases, concerns about worsening psychological stability have limited their use. More studies are required to improve the understanding on the relationship between psychodermatology disorders with inositol.
MI and its derivative IP6 have been demonstrated to be involved in a wide range of physiological and pathological settings, including cell cycle progression, apoptosis, and differentiation.36 Ins inhibits RB protein phosphorylation, preventing cell cycle progression through the RB/E2F pathway. It was also proposed that MI significantly reduces PI3K/Akt pathway activity.37 Downstream inhibition of molecular pathways supporting epithelial-mesenchymal transition prohibits possible transformation of cells into aggressive carcinomas.38 Ins has been shown to inhibit the proliferation of other types of cancer in vitro, including breast cancer, colon cancer, and lung cancer. However, its efficacy for melanoma requires further investigation.
There are wide variety of isoforms, dosing, and treatment duration employed among the included studies. Out of a total of 371 patients included in this study, mild gastrointestinal distress was reported in 11 patients, and headaches were reported in 3 patients. None of the adverse events were severe and they usually wore off with continual use. However, Seedat et al,23 reported that psychiatric patients are more prone to neurological side effects.
Conclusions
Integrative treatment for dermatology is on the rise. Our study highlighted the existing utilities of Ins in acne and hirsutism. Limited studies were available to demonstrate utility in dermatoses such as SD, HS, psoriasis, trichotillomania, and melanoma, which highlights the need for future well-designed clinical studies on alternative and adjunctive treatments are required to provide additional evidence to the field.
Funding sources
This article has no funding source.
Conflicts of Interest
The authors have no conflict of interest to declare.