1. Introduction
The skin serves as a critical barrier against microbial invasion, providing both physical and immunological defenses against pathogens. When this barrier is compromised due to injury, disease, or environmental factors, it creates an environment conducive to microbial colonization and infection, sometimes necessitating therapeutic intervention.1
Topical antibacterial agents offer several advantages over systemic treatments in managing skin infections and colonization. These include targeted delivery to the site of infection, minimal systemic absorption, and potentially reduced risk of contributing to widespread antimicrobial resistance. Moreover, topical applications can achieve higher local drug concentrations than are typically possible with systemic administration, potentially enhancing efficacy against resistant organisms.2
The landscape of topical antibacterial therapy in dermatology is undergoing rapid evolution, shaped by several factors. A primary driver of this change is the increasing prevalence of antibiotic-resistant organisms, particularly methicillin-resistant Staphylococcus aureus (MRSA), which poses significant challenges in both healthcare and community settings. Simultaneously, there is a growing understanding of the skin microbiome and its crucial role in maintaining skin health and influencing disease processes, prompting a shift towards more microbiome-conscious treatment approaches. Advancements in drug delivery technologies are enabling the development of more effective and targeted treatments, potentially improving efficacy while minimizing side effects. Furthermore, the field is witnessing the emergence of novel antimicrobial compounds and strategies, including biological agents and repurposed molecules, which offer new possibilities for addressing complex dermatological infections. These converging factors are reshaping the approach to topical antibacterial therapy in dermatology, necessitating ongoing adaptation and innovation in clinical practice. This review examines current and emerging topical antibacterial strategies in dermatology, focusing on mechanisms of action, clinical applications, recent developments, and future directions. We explore traditional antiseptics and antibiotics, as well as cutting-edge approaches such as antimicrobial peptides, nanotechnology-based delivery systems, and microbiome-modulating therapies.
2. Antiseptics
Antiseptics play a crucial role in topical antimicrobial therapy, offering broad-spectrum activity with a generally low risk of inducing bacterial resistance. Their non-specific mechanisms of action, often involving disruption of bacterial cell membranes or oxidative damage, contribute to their continued efficacy in the face of rising antibiotic resistance.
2.1. Chlorhexidine
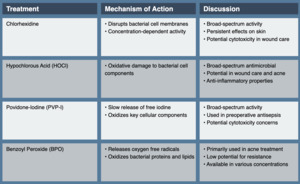
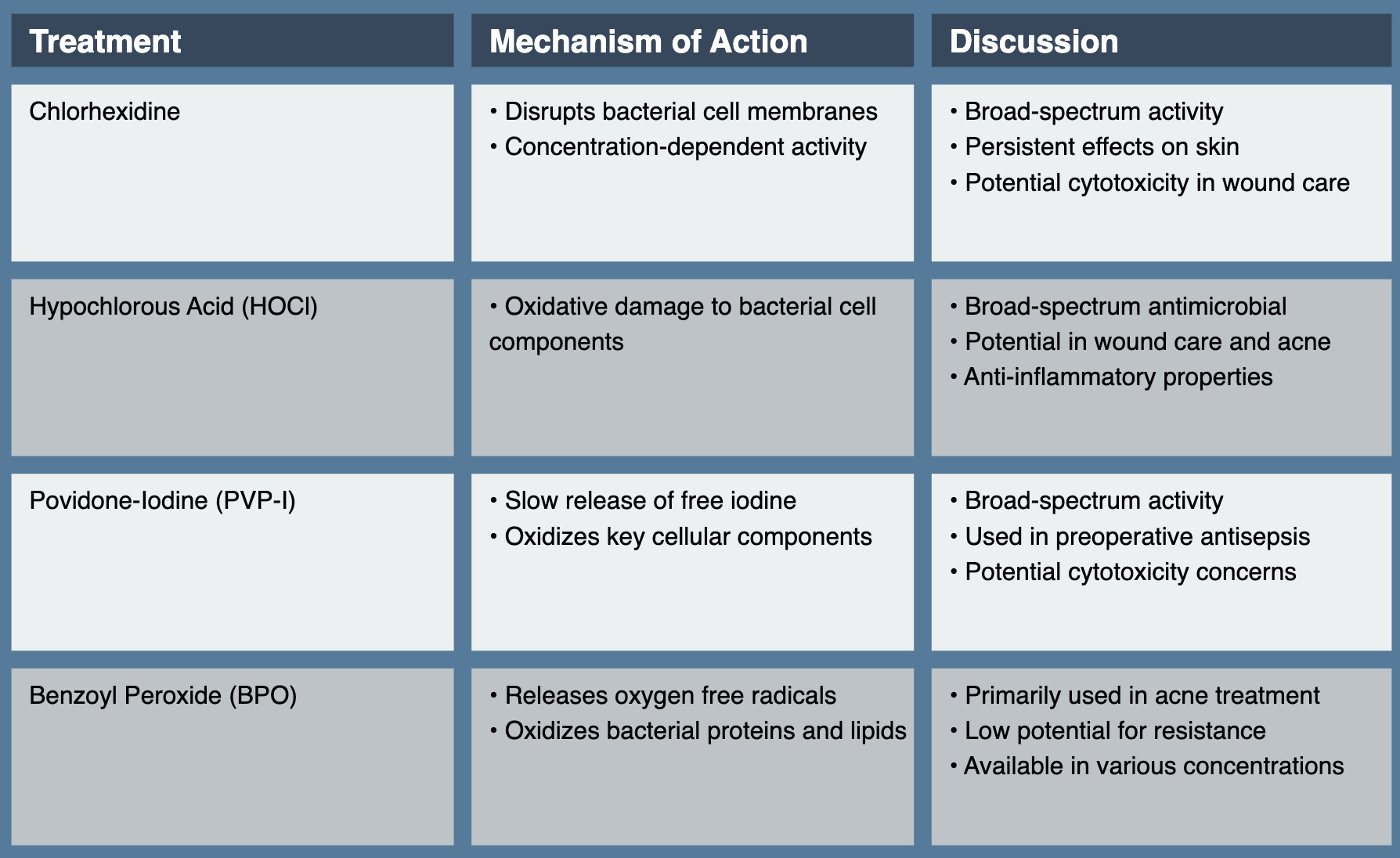
Chlorhexidine, a cationic bisbiguanide, has been a mainstay of topical antisepsis for decades. Its broad-spectrum activity against gram-positive and gram-negative bacteria, as well as some fungi and viruses, makes it valuable in various clinical scenarios. Chlorhexidine’s antimicrobial effect is primarily due to its ability to disrupt bacterial cell membranes. At low concentrations, it affects membrane integrity and osmotic equilibrium. At higher concentrations, it causes precipitation of cytoplasmic contents, leading to cell death. This concentration-dependent activity allows for both bacteriostatic and bactericidal effects, depending on the application.3
Chlorhexidine, a powerful antiseptic and disinfectant, has found widespread use in various dermatological and medical applications due to its broad-spectrum antimicrobial activity and persistent effects. In preoperative skin preparation, chlorhexidine has demonstrated significant advantages over traditional antiseptics like povidone-iodine, showing superior efficacy in reducing surgical site infections due to its persistent activity on the skin.4 While effective in reducing bacterial load in wounds, chlorhexidine’s use in wound care requires careful consideration due to potential cytotoxicity to human fibroblasts and keratinocytes, which play crucial roles in the wound healing process.5
Hand hygiene in healthcare settings has also benefited from chlorhexidine, with alcoholic chlorhexidine formulations demonstrating superior and more persistent antimicrobial activity compared to alcohol alone. This combination provides immediate germ-killing action from the alcohol, coupled with the long-lasting effects of chlorhexidine, resulting in more effective reduction of microbial flora on healthcare workers’ hands and potentially lower rates of healthcare-associated infections.6
The diverse applications of chlorhexidine in dermatology and related fields highlight its importance as a versatile tool in medical practice, though its use must always be balanced against potential risks and considerations specific to each application. Recent research has focused on optimizing chlorhexidine formulations to enhance efficacy and reduce adverse effects. One promising area of study involves nano formulations—encapsulated, incorporated, or structured at the nanometer scale (1 to 100 nanometers) to enhance efficacy, stability, and controlled release properties— where chlorhexidine-loaded nanoparticles have demonstrated potential in providing sustained antimicrobial activity while potentially reducing cytotoxicity.7 Another avenue of investigation explores combination therapies, with researchers observing synergistic effects when combining chlorhexidine with other antimicrobial agents. For instance, the combination of chlorhexidine with silver sulfadiazine has shown improved outcomes in burn wound management.8 Additionally, addressing the challenge of bacterial biofilms in chronic wound management has become a significant focus. Studies are now exploring various strategies to enhance chlorhexidine’s efficacy against these resilient bacterial communities, which pose a considerable obstacle in wound healing.9
2.2. Hypochlorous Acid
Hypochlorous acid (HOCl), a key component of the innate immune response produced by neutrophils as well as the active antimicrobial agent in household bleach, has gained attention as a topical antiseptic due to its broad-spectrum antimicrobial activity and potential to disrupt biofilms. HOCl exerts its antimicrobial effect through oxidative damage to bacterial cell components, including cell membranes, proteins, and DNA. Its small molecular size and neutral charge at physiological pH allow for rapid penetration into microbial cells.10 The use of dilute bleach baths, which produce hypochlorous acid in solution, has been explored in the management of atopic dermatitis.11 Notably, subsequent studies have demonstrated that dilute household bleach baths are not concentrated enough to be antimicrobial. Instead, they appear to have anti-itch, anti-inflammatory, and barrier-repairing properties that contribute to their therapeutic effect.12
Stabilized HOCl formulations have also demonstrated promising results in various dermatological applications. In the realm of wound care, HOCl solutions have shown efficacy in reducing bacterial load and promoting healing in chronic wounds, offering a potential alternative to conventional antimicrobial treatments.13
The applications of HOCl extend to common skin conditions such as acne vulgaris. Stabilized HOCl spray effectively was found to reduce inflammatory acne lesions and improve overall skin appearance, suggesting its potential as a novel acne treatment.14 Furthermore, HOCl-based therapies have shown promise in managing atopic dermatitis, another chronic inflammatory skin condition.15 While the exact mechanisms are still being studied, these formulations may work primarily through anti-inflammatory effects and barrier repair, similar to dilute bleach baths. Any antimicrobial effects, if present, would likely be due to the higher concentrations of HOCl in these stabilized formulations compared to dilute bleach baths.
Research into HOCl as a topical antiseptic has accelerated in recent years, with several key areas of focus emerging. Studies have demonstrated HOCl’s ability to penetrate and disrupt bacterial biofilms, a significant advantage in managing chronic infections.16 Beyond its direct antimicrobial activity, HOCl has shown potential to modulate the inflammatory response, which may contribute to its therapeutic effects in conditions like atopic dermatitis.17 Ongoing research is also exploring novel delivery systems to develop stable, long-lasting HOCl formulations, including hydrogels and nanoencapsulated forms.18
2.3. Povidone-Iodine
Povidone-iodine (PVP-I), a complex of polyvinylpyrrolidone and elemental iodine, has been a staple of topical antisepsis for decades. Its broad-spectrum activity against bacteria, fungi, viruses, and some bacterial spores makes it valuable in various clinical scenarios. PVP-I’s antimicrobial activity is primarily due to the slow release of free iodine, which oxidizes key cellular components such as nucleotides, fatty acids, and thiol groups in proteins. This multi-target approach contributes to its broad spectrum of activity and low potential for resistance development.19
Povidone-iodine (PVP-I) is widely used in dermatology and related fields, with applications spanning various areas of skin care and treatment. In preoperative skin antisepsis, PVP-I remains a standard option for surgical site preparation. However, some studies suggest that chlorhexidine may be superior in certain contexts, leading to ongoing debates about optimal preoperative antiseptic choices.20 Recently, a large study of 1,598 patients demonstrated the non-inferiority of PVP-I compared to chlorhexidine as a preoperative skin antiseptic, a finding that could change World Health Organization guidance given the lower cost of PVP-I.21,22
PVP-I also plays a role in wound care, where it is effective in reducing bacterial load. Nevertheless, concerns have arisen regarding its potential cytotoxicity and the possibility of impaired wound healing with prolonged use. These concerns have prompted a reevaluation of PVP-I’s role in chronic wound management, with healthcare providers increasingly considering alternative antiseptic options for long-term wound care.23 Despite these limitations, PVP-I continues to find utility in specific dermatological conditions. For instance, short-term use of PVP-I baths has demonstrated efficacy in managing infected eczematous dermatitis, offering a targeted approach for controlling bacterial overgrowth in eczema patients.24
Recent research has focused on optimizing PVP-I formulations and exploring new applications to address some of these concerns and expand its utility. Encapsulation of PVP-I in liposomes has shown promise in reducing irritation while maintaining antimicrobial efficacy, potentially offering a more skin-friendly alternative to traditional formulations.25 Studies have also explored the efficacy of lower concentration PVP-I preparations, which may offer a better balance between antimicrobial activity and tissue toxicity.26 Furthermore, the COVID-19 pandemic has renewed interest in PVP-I’s antiviral properties, with studies exploring its potential in reducing viral transmission.27
2.4. Benzoyl Peroxide
Benzoyl peroxide (BPO) has been a mainstay in acne treatment for over six decades. Its ability to reduce Cutibacterium acnes without inducing bacterial resistance has ensured its continued relevance in an era of increasing antimicrobial stewardship. BPO’s antibacterial activity is primarily due to its oxidizing properties. Upon application to the skin, it decomposes to release oxygen free radicals, which oxidize bacterial proteins and lipids, leading to cell death. This nonspecific mechanism contributes to its low potential for inducing bacterial resistance.28
As a monotherapy, BPO has demonstrated effectiveness in treating mild to moderate acne. It is available in a range of concentrations, typically from 2.5% to 10%, allowing for tailored treatment based on individual patient needs and skin sensitivity.29 BPO’s utility extends beyond monotherapy, as it is frequently employed in combination therapies. Combining BPO with topical antibiotics or retinoids has shown enhanced efficacy in acne treatment. Moreover, this combination approach helps mitigate the risk of developing antibiotic resistance.30
Recent research has focused on optimizing benzoyl peroxide (BPO) formulations and exploring new applications to enhance its efficacy in acne treatment. Novel delivery systems, such as micro sponge technology and nanoparticle formulations, have been developed to enhance skin penetration and reduce irritation, potentially improving patient tolerance and adherence.31 The development of fixed-dose combination products, particularly those combining BPO with adapalene or clindamycin, has shown enhanced efficacy in managing acne vulgaris, offering more convenient and potent treatment options for patients.32 Researchers have also explored the potential of BPO as a photosensitizer in photodynamic therapy for acne, which could potentially enhance its antibacterial effects and provide an alternative approach for treatment-resistant cases.33 However, recent safety concerns have emerged, particularly regarding the presence of benzene in certain BPO products. BPO is known to have a bleaching effect on towels and clothing, which is consistent across all formulations. These issues have highlighted the need for rigorous quality control in manufacturing processes and throughout distribution to ensure the safety and efficacy of BPO formulations.34 (See Table 1.)
3. Antibiotics
Topical antibiotics play a crucial role in managing various dermatological conditions, offering targeted therapy with minimal systemic absorption. However, the rising prevalence of antibiotic-resistant organisms presents a significant challenge, necessitating judicious use and the development of novel agents and strategies.
3.1. Mupirocin
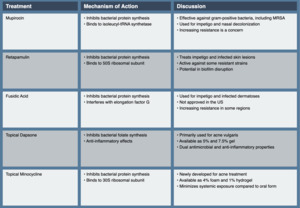
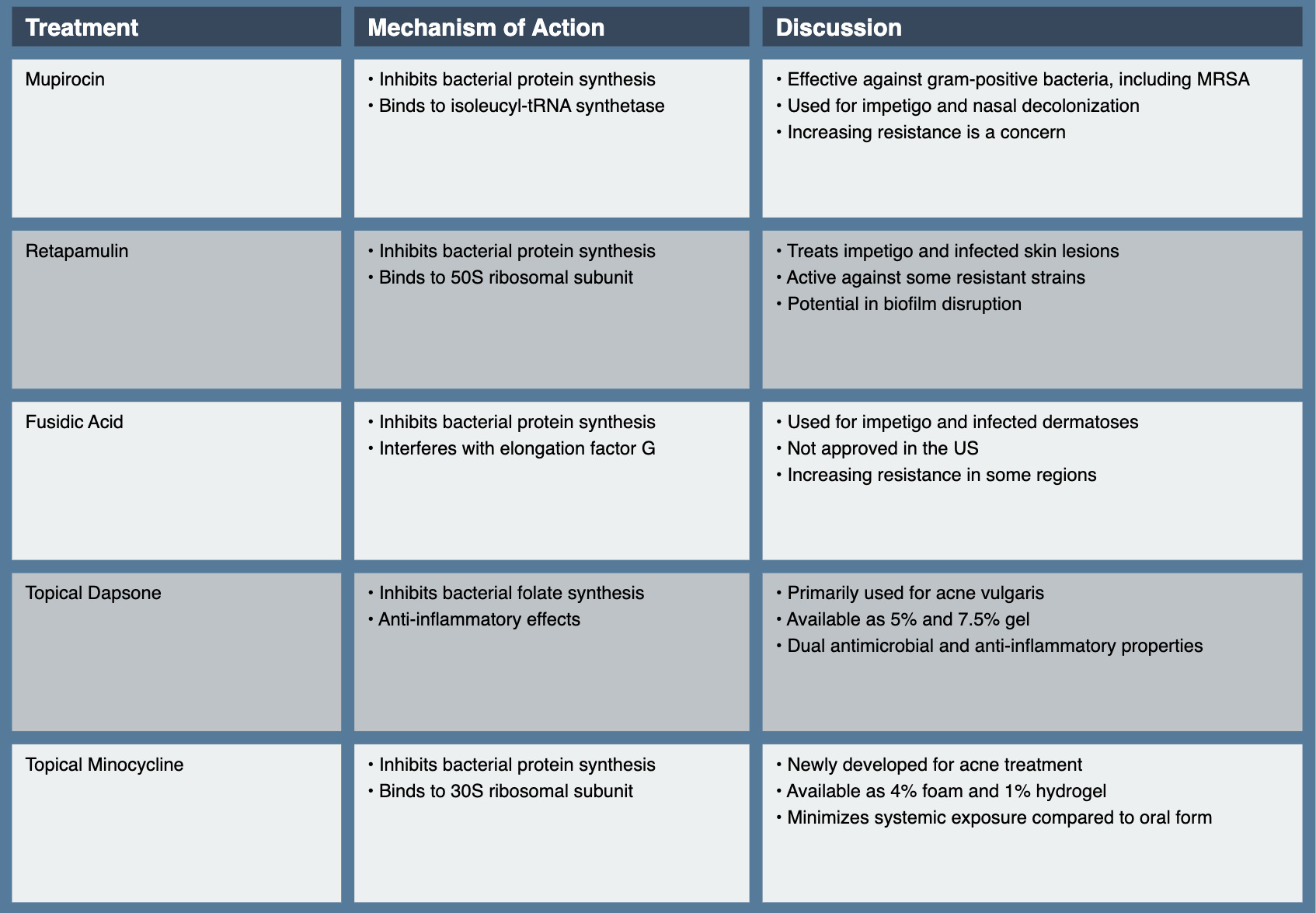
Mupirocin, derived from Pseudomonas fluorescens, has been a cornerstone of topical antibiotic therapy in dermatology since its introduction in the 1980s. Its unique mechanism of action and efficacy against gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), have made it valuable in treating various skin infections. Mupirocin inhibits bacterial protein synthesis by competitively binding to isoleucyl-tRNA synthetase, preventing the incorporation of isoleucine into nascent peptide chains. This mechanism is distinct from that of most other antibiotics, contributing to mupirocin’s low potential for cross-resistance.35
Mupirocin has found widespread application in various areas of dermatology due to its potent antibacterial properties. As a first-line treatment for impetigo, mupirocin has demonstrated superior efficacy compared to oral antibiotics in many cases, making it a preferred topical option for this common skin infection.36 Beyond its role in treating superficial skin infections, mupirocin has proven highly effective in nasal decolonization strategies. When applied intranasally, it successfully eradicates Staphylococcus aureus carriage, playing a crucial role in preventing surgical site infections and managing recurrent skin infections.37
The utility of mupirocin extends to the management of secondary skin infections as well. It is frequently employed in treating secondarily infected dermatoses, such as cases of infected eczema, where it helps control bacterial overgrowth without the need for systemic antibiotics. These diverse applications highlight mupirocin’s importance as a versatile topical antibiotic in dermatological practice, offering targeted antimicrobial activity across a range of skin conditions.
While mupirocin remains a valuable agent in dermatological practice, several challenges have emerged that threaten its long-term efficacy. Of particular concern is the increasing rate of mupirocin resistance, especially in healthcare settings. High-level resistance, mediated by the mupA gene, poses a significant threat to the continued effectiveness of this antibiotic.38 In response to these challenges, ongoing research is focused on developing novel mupirocin formulations. Nanoparticle-based delivery systems are being explored as a means to enhance skin penetration and potentially overcome some resistance mechanisms, offering hope for improved efficacy.39 Additionally, studies are investigating the potential of combination therapies, exploring the synergistic effects of combining mupirocin with other antimicrobial agents. These combination approaches aim to enhance overall efficacy and reduce the risk of resistance development, potentially extending the useful lifespan of mupirocin in clinical practice.40
3.2. Retapamulin
Retapamulin, a semisynthetic pleuromutilin derivative, represents a novel class of topical antibiotics. Approved by the FDA in 2007, it offers a new mechanism of action in the topical antibiotic armamentarium. Retapamulin inhibits bacterial protein synthesis by selectively binding to the 50S subunit of the bacterial ribosome at a site distinct from that of other macrolides. This unique mechanism allows it to maintain activity against many bacterial strains resistant to other topical antibiotics.41
Retapamulin has found its primary application in the treatment of impetigo and secondarily infected traumatic skin lesions. Clinical trials have demonstrated the efficacy of retapamulin in treating impetigo caused by Staphylococcus aureus and Streptococcus pyogenes, including some methicillin-resistant strains, establishing it as a valuable topical antibiotic option for this common skin infection.42
While not officially approved by the FDA for this indication, studies suggest that retapamulin may also have potential efficacy in managing secondarily infected dermatoses. Research has indicated possible benefits in treating secondarily infected eczema and other dermatological conditions where bacterial overgrowth complicates the primary skin disorder.43 These applications highlight retapamulin’s role as a targeted antimicrobial agent in dermatology, offering an alternative treatment option for specific skin infections and potentially expanding its utility to a broader range of dermatological conditions.
Research into retapamulin continues to expand our understanding of its potential applications and efficacy. In vitro studies have demonstrated retapamulin’s ability to penetrate and disrupt Staphylococcus aureus biofilms, suggesting potential utility in managing chronic or recalcitrant infections that are often challenging to treat with conventional antibiotics.44 Investigations into potential synergistic effects when combining retapamulin with other topical agents are ongoing, aiming to enhance its therapeutic efficacy and broaden its clinical applications.45 Crucially, ongoing surveillance of retapamulin susceptibility patterns is being conducted to preserve its long-term efficacy. To date, resistance rates remain low, likely due to retapamulin’s unique mechanism of action and its relatively limited use in clinical practice.46 This continued monitoring is essential to maintain retapamulin’s effectiveness and guide appropriate prescribing practices.
3.3. Fusidic Acid
Fusidic acid, while not approved for use in the United States, is widely used in many other countries for the topical treatment of skin infections. Its unique structure and mechanism of action make it an important option in the topical antibiotic arsenal. Fusidic acid inhibits bacterial protein synthesis by interfering with elongation factor G (EF-G) on the ribosome. It prevents the release of EF-G from the ribosome after GTP hydrolysis, effectively stalling the elongation phase of protein synthesis.47
Fusidic acid finds application in various dermatological scenarios, demonstrating its versatility as a topical antibiotic. In the treatment of impetigo, topical fusidic acid has shown significant efficacy, with outcomes comparable to those achieved with oral antibiotics in many cases.48 This makes it a valuable option for managing this common skin infection, potentially reducing the need for systemic antibiotic use. Beyond impetigo, fusidic acid has also proven useful in managing secondarily infected dermatoses. It is frequently employed in the treatment of secondarily infected eczema and other inflammatory skin conditions where bacterial overgrowth complicates the primary disorder.49 The ability of fusidic acid to address these secondary infections can help in managing the overall condition more effectively, highlighting its importance in the dermatologist’s arsenal of topical treatments.
While fusidic acid remains an important topical antibiotic in dermatology, several challenges and areas of research have emerged that warrant attention. A primary concern is the increasing prevalence of fusidic acid resistance, particularly in Staphylococcus aureus. Resistance rates show geographical variation and are influenced by local prescribing practices, highlighting the need for judicious use and ongoing surveillance.50 To address these challenges and enhance therapeutic outcomes, researchers have explored combination therapies. Studies have investigated the efficacy of combining fusidic acid with other topical agents, such as corticosteroids, particularly in managing conditions like infected eczema.51 Concurrently, ongoing research is focused on elucidating the molecular mechanisms underlying fusidic acid resistance. This deeper understanding of resistance pathways may inform strategies to preserve the long-term efficacy of fusidic acid and guide the development of novel approaches to overcome resistance.52
3.4. Topical Dapsone
Dapsone, traditionally used as a systemic agent for leprosy and other conditions, has found an expanded role in dermatology with the development of a topical formulation. Its dual antimicrobial and anti-inflammatory properties make it somewhat unique among topical antibiotics. Dapsone’s antimicrobial activity is attributed to its inhibition of bacterial folate synthesis, like sulfonamides. However, its anti-inflammatory effects, which are particularly relevant in its topical applications, are believed to result from inhibition of myeloperoxidase and other enzymes involved in neutrophil function.53
Topical dapsone is FDA-approved as a 5% and 7.5% gel for acne treatment.54 Research into topical dapsone continues to expand our understanding of its applications and mechanisms, driving improvements in its clinical use. Studies have focused on optimizing treatment regimens, with recent data suggesting comparable efficacy between once-daily and twice-daily application of the 7.5% formulation, potentially improving patient adherence.55 Mechanistic studies are exploring the molecular pathways underlying dapsone’s anti-inflammatory effects in the skin, which may inform its use in other inflammatory dermatoses beyond acne.56 In the realm of drug delivery, researchers have explored nanoparticle-based formulations to enhance skin penetration, potentially expanding topical dapsone’s therapeutic applications to a broader range of skin conditions.57
3.5. Topical Minocycline
Minocycline, a broad-spectrum tetracycline antibiotic, has long been used systemically for various dermatological conditions, particularly acne vulgaris. However, concerns about systemic side effects and the development of antibiotic resistance have led to the development of topical formulations. Topical minocycline represents a novel approach to delivering this well-established antibiotic directly to the skin, potentially offering the benefits of minocycline while minimizing systemic exposure.
Minocycline’s mechanism of action involves inhibition of bacterial protein synthesis by binding to the 30S ribosomal subunit. Additionally, it possesses anti-inflammatory properties, which are particularly beneficial in managing inflammatory skin conditions like acne and rosacea. The development of topical formulations has required innovative approaches to overcome the challenges of delivering this traditionally oral medication through the skin barrier.58
Developments in topical minocycline formulations have shown promising results. A 4% minocycline foam has demonstrated efficacy in treating moderate to severe acne vulgaris. In a phase 3 randomized, double-blind, vehicle-controlled study, this formulation significantly reduced both inflammatory and non-inflammatory lesions compared to the vehicle. The study also reported a favorable safety profile, with predominantly mild local adverse events.59 Another formulation, a 1% minocycline hydrogel, has also shown efficacy in acne treatment. A previous investigation conducted a 12-week, randomized, double-blind, vehicle-controlled study that demonstrated significant reductions in inflammatory lesions compared to the vehicle, with minimal systemic absorption and a favorable local tolerability profile.60
The development of topical minocycline formulations addresses several challenges associated with oral minocycline use. By delivering the antibiotic directly to the skin, these formulations aim to reduce systemic exposure and potentially decrease the risk of adverse effects such as dizziness, photosensitivity, and tooth discoloration. Moreover, the targeted delivery may help mitigate concerns about the development of antibiotic resistance.61
Research into topical minocycline continues to evolve, with several key areas of focus emerging. Studies are exploring the long-term efficacy and safety of these formulations, which will be crucial for understanding their place in chronic acne management. The development of novel delivery systems for minocycline is another area of active research. For instance, previous studies explored the potential of minocycline-loaded nanoliposomes for enhanced skin penetration and targeted delivery to the pilosebaceous unit, which could further improve efficacy in acne treatment.62 While topical minocycline formulations show promise, several challenges and considerations remain. The potential for local irritation or sensitization needs ongoing evaluation, particularly with long-term use. Additionally, while topical application may reduce systemic exposure, the impact on the development of antibiotic resistance still requires careful monitoring and study.63 (See Table 2.)
4. Antibacterial Enzymes
The rise of antibiotic resistance has spurred interest in alternative antimicrobial strategies, including the use of bacteriophage-derived enzymes. Endolysins have emerged as promising candidates for topical antimicrobial therapy in dermatology. Endolysins are bacteriophage-encoded enzymes that hydrolyze the peptidoglycan layer of bacterial cell walls leading to cell lysis. Their high specificity for target bacteria and low likelihood of resistance development makes them attractive alternatives to traditional antibiotics. Endolysins typically consist of two functional domains: a catalytic domain that cleaves specific bonds in the peptidoglycan cell wall and a binding domain that ensures high specificity for the target bacteria. This dual-domain structure allows for rapid and specific bacterial killing without affecting commensal flora or human cells.64
While still largely in the experimental stage, several endolysin-based products have shown promise in dermatological applications. One recombinant form of the SAL-1 endolysin, has demonstrated efficacy against S. aureus in patients with S. aureus-related dermatoses. A randomized, placebo-controlled trial by Totté et al. Showed significant reduction in S. aureus load on lesional skin without affecting other commensal bacteria.65 A recent open-label study evaluating a cream containing S. aureus-targeting endolysin technology in 43 patients with mild-to-moderate atopic dermatitis demonstrated significant improvements in disease severity, with SCORing Atopic Dermatitis (SCORAD) reductions of 43% and 68% at 7 and 14 days, respectively. This study not only highlights the potential efficacy of endolysin-based therapies in managing inflammatory skin conditions associated with S. aureus overgrowth but also suggests good tolerability across a wide age range, from infants to adults.66
Research into endolysin-based therapies is rapidly advancing. Becker et al. Developed a chimeric endolysin with enhanced activity against S. aureus and improved stability under physiological conditions, potentially expanding its therapeutic applications.67 Studies are exploring the potential of combining endolysins with traditional antibiotics to enhance overall antimicrobial efficacy and potentially overcome some resistance mechanisms.68 Additionally, endolysins have shown promise in disrupting bacterial biofilms, a significant challenge in managing chronic skin infections.69
While endolysin-based therapies show great promise, several challenges remain. As protein-based therapies, endolysins have the potential to elicit an immune response. Optimizing the stability and skin penetration of endolysin formulations is crucial for their successful clinical application. As a novel class of antimicrobials, endolysins present unique regulatory challenges that need to be addressed. While resistance development is considered unlikely due to the essential nature of the peptidoglycan target, ongoing surveillance will be crucial as these therapies enter widespread use.
5. Botanicals and Natural Treatments
The growing interest in “natural” products and the search for novel antimicrobial compounds has led to increased research into botanical and other natural treatments for dermatological conditions. While many of these agents show promise in vitro or in small clinical studies, rigorous large-scale trials are often lacking.
5.1. Honey
Medical-grade honey, particularly Manuka honey from New Zealand, has gained attention for its antimicrobial and wound-healing properties in dermatological applications. The antibacterial activity of honey is attributed to multiple factors. Its high sugar content and low pH create an environment inhospitable to bacterial growth. Additionally, the enzyme glucose oxidase in honey produces hydrogen peroxide, contributing to its antimicrobial effects. Manuka honey contains non-peroxide antibacterial factors such as methylglyoxal. The presence of antimicrobial peptides like bee defensin-1 further enhances honey’s antibacterial properties.70
Honey has demonstrated potential in various dermatological applications such as in wound healing and management of skin conditions. A systematic review by Moore et al. found that honey-based dressings were more effective than conventional dressings in healing partial-thickness burns and infected post-operative wounds.71 This suggests a possible role for honey in wound care management. Beyond wound healing, honey has shown promise in managing atopic dermatitis (AD). A randomized controlled trial by Alangari et al. demonstrated topical application of Manuka honey significantly reduced Staphylococcus aureus colonization and improved SCORing Atopic Dermatitis (SCORAD) scores in patients with AD.72 These findings highlight the potential of honey as a therapeutic option for managing inflammatory skin conditions with associated bacterial colonization.
Efforts are underway to develop standardized honey-based products with consistent antimicrobial activity, which would ensure more reliable clinical outcomes.73 This standardization is crucial for the broader acceptance and use of honey-based treatments in clinical practice. Additionally, studies are exploring the potential synergistic effects of combining honey with other topical treatments, which may lead to more effective therapeutic strategies.74 Recent research has also focused on honey’s ability to disrupt bacterial biofilms, a property that may be particularly valuable in managing chronic wounds.75 Biofilms represent a significant challenge in wound care, and honey’s potential to address this issue could significantly enhance its clinical utility. The growing body of research on medical-grade honey in dermatology underscores its potential as a natural, multi-faceted therapeutic agent. Its combined antimicrobial, anti-inflammatory, and wound-healing properties make it a promising option for multiple skin conditions.
5.2. Coconut Oil
Virgin coconut oil has attracted interest within dermatology for its potential antimicrobial and moisturizing properties. The antimicrobial activity of coconut oil is primarily attributed to its medium-chain fatty acids, particularly lauric acid and its derivative, monolaurin. These compounds have been shown to disrupt bacterial cell membranes, leading to cell death.76 This mechanism of action suggests that coconut oil could serve as a natural alternative or complement to traditional antimicrobial treatments in certain dermatological conditions.
Coconut oil has demonstrated promise in several dermatological contexts, particularly in the management of atopic dermatitis (AD) and potentially in acne treatment. Evangelista et al.77 and Verallo-Rowell et al. Found that virgin coconut oil was superior to mineral oil in improving AD symptoms and reducing Staphylococcus aureus colonization in AD patients. This finding suggests that coconut oil could be a valuable addition to AD management strategies, potentially offering both antimicrobial and moisturizing benefits. In the context of acne vulgaris, an in vitro study by Nakatsuji et al. Demonstrated potent antimicrobial activity of lauric acid against C. acnes.78 While clinical studies are needed to confirm these effects in vivo, this research suggests potential applications of coconut oil or its components in acne treatment.
Studies have explored various formulations of coconut oil to enhance its antimicrobial efficacy and skin penetration.79 This formulation optimization could lead to more effective and targeted coconut oil-based treatments for skin conditions. Additionally, investigations into combining coconut oil with other active ingredients for enhanced therapeutic effects are ongoing.80 Further studies are also examining coconut oil’s potential to favorably modulate the skin microbiome beyond its direct antimicrobial effects.81 This avenue of research is particularly intriguing as it suggests that coconut oil might have broader impacts on skin health by influencing the balance of microorganisms on the skin. Understanding these microbiome-modulating effects could open new possibilities for using coconut oil in maintaining skin health and treating various dermatological conditions.
5.3. Essential Oils
Essential oils, including tea tree, thyme, and oregano, have demonstrated antimicrobial properties in vitro and in small clinical studies, attracting interest in their potential dermatological applications. The antimicrobial activity of essential oils is typically attributed to their complex mixture of terpenes and other organic compounds. These components can disrupt bacterial cell membranes and interfere with cellular processes, providing a multi-faceted approach to combating microbial growth.82
While clinical evidence is limited, several essential oils have shown potential in dermatological applications. Tea tree oil, for instance, has demonstrated efficacy in acne treatment. A randomized controlled trial by Enshaieh et al. Found that 5% tea tree oil gel was effective in reducing both inflamed and non-inflamed acne lesions.83 This suggests that tea tree oil could potentially serve as a natural alternative or complement to conventional acne treatments. Thyme oil has also shown promise, with in vitro studies demonstrating potent activity against Staphylococcus aureus, including methicillin-resistant strains.84 These findings highlight the potential of essential oils in addressing antibiotic-resistant bacterial infections, a growing concern in dermatology.
Despite their potential, the use of botanical and natural treatments in dermatology faces several challenges that need to be addressed. Standardization is a crucial issue, as ensuring consistent potency and quality of botanical extracts is essential for their acceptance in mainstream medical practice. The regulatory pathway for botanical products can be complex, particularly when making specific health claims, which may hinder their development and marketing. Additionally, there is a potential for sensitization, as some botanical ingredients can cause allergic reactions or contact dermatitis in sensitive individuals. This emphasizes the need for careful patient selection and monitoring when using these treatments. The potential for interactions between botanical treatments and conventional medications also needs to be thoroughly investigated to ensure safe and effective use.
Looking to the future, several key research directions should be pursued to advance the field of botanical antimicrobials in dermatology. Conducting large-scale, randomized controlled trials is essential to establish the efficacy and safety of these treatments in various dermatological conditions. The development of standardized, pharmaceutical-grade formulations of promising botanical extracts would help address concerns about consistency and quality. Investigating potential synergies between botanical antimicrobials and conventional treatments could lead to more effective combination therapies. Finally, elucidating the molecular mechanisms underlying the antimicrobial effects of botanical compounds would provide a stronger scientific foundation for their use and could guide the development of more targeted and effective treatments. As research in this area progresses, botanical and natural treatments may offer valuable alternatives or complements to conventional antimicrobial therapies in dermatology.
5.4. Other Botanicals
Recent research has explored the potential of various botanical extracts in dermatological applications, with some showing promise for their antimicrobial and anti-inflammatory properties. One such example is Gotu Kola (Centella asiatica). Gotu Kola, an herb long used in traditional medicine, has also garnered attention for its potential dermatological applications. Centella asiatica has been to promote wound healing through several mechanisms, including stimulation of collagen production and antioxidant effects.85 Additionally, some research suggests that Gotu Kola may have antimicrobial properties, though more studies are needed to confirm these effects in clinical settings. While botanicals show promise, it is important to note that, as with all natural products, standardization of extracts and rigorous clinical testing are necessary to establish their efficacy and safety. Furthermore, potential interactions with other medications and long-term effects need to be carefully evaluated before these botanicals can be widely recommended for dermatological use. (See Table 3.)
6. Future Directions and Emerging Technologies
The field of topical antibacterial therapy in dermatology continues to evolve, driven by advances in understanding of skin biology, microbiology, and drug delivery technologies. Several promising areas of research are likely to shape the future of topical antimicrobial strategies.
6.1. Antimicrobial Peptides (AMPs)
Antimicrobial Peptides (AMPs), small proteins produced as part of the innate immune response, represent a promising frontier in topical antimicrobial therapy for dermatological conditions. AMPs typically act by disrupting bacterial cell membranes, a mechanism that makes the development of resistance less likely compared to traditional antibiotics. Many AMPs also possess immunomodulatory properties, potentially offering dual benefits in managing inflammatory skin conditions with a microbial component.86 This unique combination of antimicrobial and immunomodulatory effects makes AMPs particularly intriguing for dermatological applications.
Researchers are developing synthetic peptides that mimic natural AMPs but with enhanced stability and activity. For example, a study by de la Fuente-Núñez et al. Reported the development of a synthetic peptide with potent activity against multidrug-resistant pathogens, including MRSA.87 This approach could potentially address some of the limitations of natural AMPs while retaining their broad-spectrum antimicrobial activity. To overcome some of the challenges associated with peptide-based therapies, researchers are also developing AMP-inspired small molecules. These compounds aim to retain the broad-spectrum activity of AMPs while improving stability and reducing production costs.88 This strategy could make AMP-based therapies more feasible for widespread clinical use by addressing issues of stability and cost-effectiveness.
Studies are exploring the potential synergistic effects of combining AMPs with conventional antibiotics. A study by Tan and Johari demonstrated enhanced efficacy against biofilm-forming bacteria when combining AMPs with traditional antibiotics.89 This approach could potentially lead to more effective treatments for challenging infections, such as those involving biofilms, which are often resistant to conventional antibiotic monotherapy.
While AMPs show great promise, several challenges need to be addressed before they can be widely adopted in clinical practice. Stability is a significant concern, as many natural AMPs are susceptible to degradation by proteases. Developing stable formulations for topical application is crucial for their effectiveness in dermatological use. The cost of production is another hurdle, as the synthesis of peptide-based therapies can be expensive. Advances in synthesis technologies and the development of AMP-inspired small molecules may help address this issue, making AMP-based treatments more economically viable. Delivery is another critical aspect that requires optimization. Ensuring adequate skin penetration and targeting to the site of infection is essential for the efficacy of AMP-based treatments. This may involve developing novel delivery systems or formulations that can effectively transport AMPs through the skin barrier to reach their intended targets.
Looking to the future, research should focus on several key areas to advance the field of AMP-based therapies in dermatology. Optimizing AMP formulations for topical use is crucial to ensure their stability and effectiveness when applied to the skin. Further exploration of combination therapies could lead to more potent and versatile treatments. Conducting large-scale clinical trials is essential to establish the efficacy and safety of AMP-based treatments in various dermatological conditions. Their unique mechanisms of action, potential for dual antimicrobial and immunomodulatory effects, and lower likelihood of inducing resistance make them attractive candidates for addressing challenging skin infections and inflammatory conditions.
6.2. Nanotechnology-Based Delivery Systems
Nanotechnology offers exciting possibilities for enhancing the delivery and efficacy of topical antibacterial agents. Various types of nanocarriers have been developed for this purpose, each with unique properties. Liposomes are phospholipid vesicles that can encapsulate both hydrophilic and hydrophobic drugs. Solid Lipid Nanoparticles (SLNs) are lipid-based carriers with a solid core at room temperature. Polymeric Nanoparticles are made from biodegradable polymers, while Nanofibers are electrospun fibers with diameters in the nanometer range. These nanocarriers offer several benefits for topical antibacterial therapy. They can enhance skin penetration, provide sustained drug release, and protect unstable compounds. There is also potential for targeted delivery to specific skin structures, such as hair follicles. Some nanoparticles, like silver nanoparticles, may even have intrinsic antimicrobial properties.
Hussain et al. developed a liposomal formulation of mupirocin that demonstrated enhanced skin penetration and antimicrobial efficacy compared to conventional mupirocin ointment in an in vitro model of infected skin.90 In another study, Tian et al. Created a topical gel containing silver nanoparticles for treating chronic wounds.91 In a small clinical trial, this nanoparticle-containing gel significantly accelerated wound healing and reduced bacterial load compared to standard care. Despite these advancements, several challenges need to be addressed before nanotechnology-based approaches can be widely adopted. The long-term safety of nanoparticles, particularly concerning potential systemic absorption and environmental impact, requires careful evaluation. Developing cost-effective methods for large-scale production of nanocarriers is crucial for their widespread use. Additionally, the unique properties of nanocarriers may necessitate new regulatory frameworks to ensure their safety and efficacy. Looking to the future, research should focus on optimizing nanocarrier formulations for specific dermatological applications.
6.3. Microbiome Modulation
As our understanding of the skin microbiome continues to grow, there is increasing interest in developing therapies that selectively modulate microbial communities rather than broadly eradicating bacteria. This shift in approach recognizes the complex ecological nature of the skin microbiome and its importance in maintaining skin and whole person health. Several strategies are being explored to achieve this targeted modulation, including prebiotic therapies, probiotic therapies, and the use of quorum sensing inhibitors. Prebiotic therapies involve the use of substances that selectively promote the growth of beneficial bacteria. Probiotic therapies, on the other hand, involve the direct application of beneficial bacteria to the skin and are widely available as oral preparations, but have had far fewer topical options thus far. Quorum sensing inhibitors are compounds that interfere with bacterial communication systems, potentially disrupting the formation of biofilms or the expression of virulence factors.
Recent developments in these microbiome-based approaches have shown promising results. In the realm of prebiotic therapy, Al-Ghazzewi et al. found that a prebiotic gel containing glucomannan oligosaccharides improved symptoms and reduced S. aureus colonization in patients with atopic dermatitis.92 Topical probiotics continue to show potential in clinical studies, with novel approaches emerging in the field. One particularly interesting development involves the use of Nitrosomonas, a genus of ammonia-oxidizing bacteria (AOB) naturally found in soil and water. These bacteria have been investigated for their potential benefits in skin health, particularly in managing inflammatory skin conditions. AOB can convert ammonia, a component of sweat, into nitrite and nitric oxide, which may have anti-inflammatory and beneficial antimicrobial properties on the skin.93
Research into quorum sensing inhibitors has yielded interesting results as well. Escobar-Muciño et al. demonstrated that a topical formulation containing the quorum sensing inhibitor baicalein reduced C. acnes colonization and improved acne symptoms in a small clinical trial.94 This approach of interfering with bacterial communication systems represents a novel strategy for managing skin conditions associated with bacterial overgrowth.
While microbiome-based approaches hold great promise, significant research is still needed to fully realize their potential. Developing a deeper understanding of the complex interactions within the skin microbiome is crucial for designing effective therapies. Ensuring the stability and viability of live bacterial formulations presents a technical challenge that needs to be addressed for probiotic therapies. There are also regulatory challenges associated with live bacterial products that need to be navigated. Long-term studies are essential to ensure the safety and efficacy of microbiome-modulating therapies. Given the dynamic, interconnected nature of the skin microbiome, it is important to understand the long-term effects of these interventions on overall skin health and microbial ecology.
Conclusion
The field of topical antibacterial therapy in dermatology is undergoing a period of rapid evolution and innovation. While traditional antiseptics and antibiotics continue to play crucial roles, emerging technologies such as antimicrobial peptides, nanotechnology-based delivery systems, new botanicals, and microbiome-modulating therapies offer new possibilities for more targeted and effective treatments. The challenges posed by antimicrobial resistance and the complex nature of many skin conditions continue to drive innovation in drug delivery, combination therapies, and microbiome-focused approaches.
As our understanding of skin microbiology and the intricate interplay between the skin barrier, microbiome, and immune system continues to advance, we can expect to see the emergence of more personalized and sustainable approaches to managing bacterial skin infections and related dermatological conditions. However, as with all medical advances, careful evaluation of both benefits and risks will be crucial as these new therapies make their way from the laboratory to clinical practice. Long-term safety studies, optimization of treatment protocols, and ongoing surveillance for resistance development will be essential to ensure the responsible development and use of these innovative therapies.
The future of topical antibacterial therapy in dermatology is likely to be characterized by a more nuanced, targeted, and holistic approach to promoting skin health and combating infection. By leveraging advances in basic science, drug delivery technology, and clinical research, we have the potential to significantly improve outcomes for patients with a wide range of dermatological conditions, from common ailments like acne and atopic dermatitis to more challenging infections and chronic wounds.