INTRODUCTION
The epithelial barrier is the first line of physical, chemical, and immunological defense against allergens, irritants, pathogens, and other environmental factors. Innate and adaptive immune responses play a role in epithelial barrier damage. Structural and functional integrity of the epithelial barrier is critical as damaged epithelial barriers are linked to inflammatory and autoimmune conditions. A dysfunctional epithelial barrier leads to allergen sensitization, which plays a role in the pathogenesis of atopic conditions.1
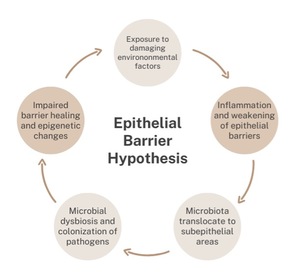
The “epithelial barrier hypothesis” proposes that genetic predisposition to epithelial barrier damage, exposure to epithelial barrier-damaging agents, and chronic epithelial inflammation are responsible for the development of allergic and autoimmune conditions. Akdis postulates that there is a linkage between epithelial barrier damaging agents and industrialization and urbanization.1 The increase in modern industrialization and urbanization is also believed to be correlated to the rise in allergen sensitization. A damaged and leaky epithelial barrier can alter the microbiome through the colonization of opportunistic pathogens, local inflammation, and decreased diversity in microbiota (Figure 1). Exposure to damaging agents can cause inflammation in the epithelia and subsequently cause translocation of microbiota. This leads to a robust immune response. Consequently, this dysregulation in immune response exacerbates inflammatory and autoimmune conditions by decreasing biodiversity and increasing microbial dysbiosis. Ultimately, this leads to an impaired epithelial barrier, one which has a decreased healing capacity and is susceptible to novel opportunistic pathogens.2
The objective of this narrative review is to better understand the scientific underpinnings of the epithelial barrier hypothesis proposed by Akdis, as well as discuss the important roles of the skin, gut, and respiratory barriers. This paper seeks to understand knowledge gaps in the link between damaged epithelia and dysbiosis, inflammation, and allergy. The link between barrier disruption and broad disease states can be used to better understand underlying etiopathogenesis. Moreover, protection of the epithelial barrier may help prevent the development of allergen sensitization. A clearer understanding of the mechanisms that affect the integrity of the skin, gut, and respiratory barriers will allow for fully informed decision making by providers as well as patients during the management of inflammatory and autoimmune conditions.
METHODS
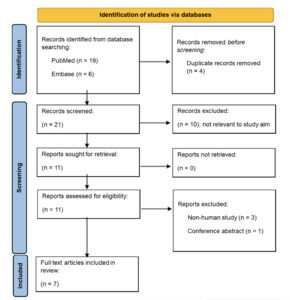
A systematic search of PubMed and Embase was performed using search terms relating to epithelial barriers and damaging agents. Initially, twenty-five articles across both databases were found. All captured titles and abstracts were screened. Duplicate articles were removed. Articles were then reviewed for relevance using inclusion exclusion criteria. Articles not relevant to the study aim were excluded. Eleven articles were then retrieved. Non-human studies and conference abstracts were excluded. A flowchart demonstrating this process is shown in Figure 2. Seven full text articles were included in the review.
Inclusion Criteria:
-
Epithelial barrier dysfunction / dysregulation / damage
-
Epithelial barrier damaging agents
Exclusion Criteria:
-
Non-human studies
-
Conference abstracts
RESULTS
PRIOR HYPOTHESES FOR ALLERGIC INCREASE
Over the past decades, many hypotheses have been proposed as explanations for increasing allergy prevalence. David Strachan proposed the “hygiene hypothesis” in 1989. He hypothesized that fewer microbial exposures early in childhood could be an explanation for the increase in allergic responses in adulthood. Recurrent microbial exposures would initiate a T-helper 1 (Th1) immune response rather than a T-helper 2 (Th2) immune response. The mechanism behind this hypothesis comprises toll-like receptors (TLRs), a component of the innate immune system. TLRs recognize bacterial endotoxins, allowing for Th1 cell activation. Th1 cells have a protective function as they utilize antiviral defenses to inhibit respiratory tract damage, and they reduce abnormal repair mechanisms responsible for mucosal hyperplasia.3
The “old friends hypothesis” proposed by Graham Rook in 2003, postulated that adequate exposure to infectious diseases was necessary for the complete development of the immune system. Rook believed that sufficient exposure to microorganisms is critical to drive expansions of components such as T regulatory cells that mediate the immune response. It was thought that failure of immunoregulation was due to a lack of microbial exposures. Similar to Strachan, Rook believed that the more diverse the microbial environment, the better equipped the immune system would be.4
The “biodiversity hypothesis” proposed by Tari Haahtela in 2019, states that the greater the diversity of microbial species, the less dominant their presence within the microbiome. This would lead to a more balanced immune system thus protecting one from allergic and inflammatory disorders. Similar to the “hygiene hypothesis,” the “biodiversity hypothesis” supports the idea that more exposure to natural environments enriches the microbiota.5
All these hypotheses posited that greater microbial diversity leads to a better regulated immune system. In recent years, it has been suggested that these hypotheses do not fully explain the rise in allergic and autoimmune conditions. We summarize some of these shortcomings below:
-
Allergic asthma is still increasing in some Asian and African countries despite relatively low hygiene standards.6
-
Increases in respiratory diseases and measles does not seem to protect against allergic diseases.7
-
Allergic diseases are increasingly seen in rural Africa, where children are exposed to relatively low hygiene conditions.7
THE BARRIER HYPOTHESIS AS PRESENTED BY AKDIS
The epithelial barrier hypothesis provides a more comprehensive understanding of environmental exposures encountered daily and the impact on biodiversity. The epithelial barrier hypothesis proposes that disrupted epithelial barriers are prone to bacterial leakage and dysbiosis. Translocation of bacteria leads to inflammation in adjacent tissues. This epithelial barrier damage is a commonality seen across a broad range of conditions—from atopic dermatitis to inflammatory bowel syndrome. Akdis discusses how the link between epithelial barrier disruption and broad disease states can be used to prevent disease. Early protection of the epithelial barrier may have beneficial effects on the prevention and development of allergen sensitization in the first few years of life.
Many environmental agents have been identified as damaging agents for epithelial barrier function.1 Some of these damaging agents include (but are not limited to): detergents, emulsifiers, and isocyanates (Table 2). Exposomes are a measure of all the environmental exposures an individual encounters in a lifetime and the impact these exposures have on one’s health. They incorporate both internal and external factors. Exposomes such as global warming, loss of biodiversity, pollution, pathogens, household cleaners, and microplastics cause epithelial cell injury, decreased microbiota diversity, bacterial translocation, allergic sensitization, and inflammation in the periepithelial area.8 Importantly, the vast majority of these substances stem from modern industrialization and urbanization. This rise in modern industrialization and urbanization is believed to be associated with the increase in allergies. Many of these substances are also present in cleaning products or personal hygiene products such as shampoos and body washes.8
BARRIER DEFECT AND THE SYSTEMIC IMMUNE RESPONSE
The epithelial barrier depends largely on the outermost layer of the epidermis, the stratum corneum. The stratum corneum consists mainly of keratinocytes and provides protection from external stimuli. Defects in the stratum corneum allow for foreign antigens, irritants, and pathogens to enter the epidermis. With stratum corneum covering most of the skin, tight junctions provide a second line of defense against the external environment. Tight junctions are composed of transmembrane proteins which are essential to the integrity of the epithelial barrier. In areas lacking stratum corneum such as hair follicles and sweat glands, tight junctions serve as the primary barrier in protecting epithelia. Impairment in tight junction expression has been shown to contribute to the barrier dysfunction observed in patients with atopic dermatitis.16 It has been recently hypothesized that the dysbiosis seen in atopic dermatitis patients may be related to isocyanate exposure. Production of diisocyanates, a type of isocyanate and an industrial toxin, rapidly increased in the mid to late 1970s. This is also when atopic dermatitis rates began to rise. Methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) are the most common industrial diisocyanates manufactured for a variety of products such as furniture cushions, nylon, paint, polyurethane, and adhesives. Many of these products are associated with an increased risk of atopic dermatitis.15
The intestinal barrier is a selectively permeable barrier, meaning it allows for the absorption of nutrients, electrolytes, and water while defending against the entry of toxins and antigens. Through desmosomes, adherens junctions, and tight junctions, the intestinal barrier maintains this selective permeability. The first line of defense is the mucous gel layer in combination with the alkali secreted by the surface epithelium. Agents which damage the mucosa such as aspirin and bile salts alter the pH gradient allowing the luminal surface of the epithelium to become more acidic.17 Disrupted intestinal barrier function has become increasingly associated with the development of autoimmune and inflammatory diseases.18 Gut barrier defects have been observed in patients with systemic lupus erythematosus as well as rheumatoid arthritis. Interactions between the gut microbes and the host were found to be significant in the development of systemic lupus erythematosus.19 Gut barrier leakiness was found to be linked to rheumatoid arthritis development via increased levels of serum zonulin as well as the migration of inflammatory cells from the gut to the joints.20 Additionally, children with atopic dermatitis often have accompanying gastrointestinal symptoms due to the disruption of the intestinal barrier integrity.21 As previously discussed, diisocyanates have been associated with dysbiosis in atopic dermatitis patients. Diisocyanates are not only strongly correlated with atopic dermatitis, but they also disrupt commensal bacteria’s therapeutic pathway. A few of the commensal strains adversely affected by diisocyanates are R. mucosa, S. epidermidis, and S. cohnii. For example, S. epidermidis enhances host ceramide production and subsequently the production of sphingomyelinases. This enhances gut barrier function. Toluene diisocyanate (TDI), one of the most common industrial diisocyanates, significantly altered S.epidermidis-induced glycerophospholipid metabolism and reduced the total ceramide accumulation in human primary keratinocyte cultures. Diisocyanates demonstrate a strong association with gut barrier dysregulation.15
The respiratory barrier provides protection against external particles by way of cilia and mucus. From the nasal cavity to the conducting bronchioles, the respiratory epithelium consists of ciliated pseudostratified columnar cells. The respiratory bronchioles and alveoli consist of simple squamous epithelial cells responsible for gas exchange and surfactant production. Airway epithelial cells serve a crucial role in preventing entry of irritants to areas where gas exchange is occurring. It has been proposed that the immune response to inhaled agents such as cigarette smoke is directed toward the airway epithelium. This is due to damage of lung epithelial cells as seen in patients with chronic pulmonary obstructive disease.22 Air pollution is another exposome that damages epithelium function via decreased IFN-gamma and Club cell (formerly known as Clara cell) secretory protein levels.23 Numerous studies have shown that respiratory epithelia is compromised in patients with asthma.24 Diisocyanates are a strongly associated variable for atopic diseases seen in children, especially those with peanut allergies, general atopy, and asthma. Isocyanic acid is a component of smoke from wildfires, tobacco smoke, and gasoline exhaust. Isocyanic acid, similar to toluene diisocyanate (TDI), induces N2 independence. This allows for the fermentation of isopropyl alcohol at the expense of essential lipid production, decreasing therapeutic benefit.15 This epithelial dysfunction can lead to aberrant immune responses which in turn leads to inflammation and exacerbation of allergen sensitization.
CONCLUSION
There is growing evidence to suggest that diseases with vastly different clinical manifestations have been linked to disrupted epithelial barriers. Epithelial damage can happen in many anatomical locations and with the complexity of immune regulation, it is vital to understand the mechanisms that affect the integrity of the skin, gut, and respiratory barriers. We believe the “epithelial barrier hypothesis” provides a compelling explanatory model for these disparate phenomena. This understanding allows clinicians and researchers to develop preventative measures and therapies to restore epithelial barrier structure and function.
Exposure to known epithelial barrier disruptors such as food emulsifiers, cigarette smoke, microplastics, and isocyanates appear to open the door to allergic sensitization by compromising barrier function. Isocyanates, in particular, have been strongly implicated with an increased risk of atopic dermatitis. From a dermatologic standpoint, isocyanates can disrupt the skin barrier, increasing its permeability and allowing allergens to penetrate. This triggers an immune response and collectively exacerbates skin inflammation. Continued research into epithelial barrier dysfunction is still necessary to develop strategies to reduce diseases associated with disrupted epithelial barriers. Biomarkers for leaky barriers, novel therapies for strengthening epithelial barrier molecules, blockage of bacteria, and pathogenic colonization are a few possible therapies to further explore.
Funding
No funding was received for any aspect of this manuscript.
Disclosure
PL reports being on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oréal, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, Verrica; reports consulting/advisory boards for Alphyn Biologics (stock options), AbbVie, Almirall, Amyris, Arcutis, ASLAN, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs (stock options), Concerto Biosci (stock options), Dermavant, Eli Lilly, Galderma, Janssen, LEO Pharma, Lipidor, L’Oréal, Merck, Micreos, MyOR Diagnostics, Regeneron/Sanofi Genzyme, Sibel Health, Skinfix, Suneco Technologies (stock options), Soteri Skin (stock options), Theraplex, UCB, Unilever, Verdant Scientific (stock options), Verrica, Yobee Care (stock options). In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member emeritus of the National Eczema Association.
KP report no relevant disclosures.