Introduction
Plasma is a state created by the application of energy to gasses which leads to reactive species in a neutral background of gas.1 The initial applications of plasma devices were centered around the heat generation aspect of creating plasma. The first plasma devices were termed “thermal” or “warm” plasma and can achieve extremely high temperatures. Thus, while they are potent bactericidal tools, they are also very damaging if applied to human tissue. “Non-thermal” or “cold” atmospheric plasma (CAP) was later developed and would be able to circumvent this issue. These “cold” devices prevent overheating the tissue by working at less than 40oC or applying the plasma in a pulsatile manner.2 Furthermore, CAP is significantly more affordable and therefore a more accessible potential therapy than warm plasma.3 CAP devices can be classified as direct, indirect, or hybrid discharge, depending on the plasma is generated. The plasma generated machines can vary in size as well as treatment length, power applied, or the area of treatment covered.
Cold atmospheric plasma has been found to have several attributes relevant to dermatology. Of particular significance, it has been shown to have both bactericidal and anti-inflammatory properties.4–7 CAP has been found to inhibit growth of both gram positive and negative bacteria in an indirect way by generating reactive oxygen species.5 Additionally, though the mechanism is not fully understood, it has been posited that CAP can upregulate the expression of anti-inflammatory cell mediators and suppress proinflammatory ones.6 (See Figure 1)
CAP has been shown to have therapeutic potential in the fields of oncology, hematology, immunology, and ophthalmology.8–11 In the cutaneous realm, CAP has been fairly well-studied in wound care. For example, patients with diabetic foot ulcers being treated with CAP as adjunctive therapy have shown significant improvements in the reduction of bacterial load and infections, wound size reduction, and overall enhanced wound healing.12,13 CAP has been shown to aid in the healing of venous ulcers by improving microcirculation in the area.14–16
Beyond wounds, animal and in vitro studies are slowly emerging that examine CAP in other dermatologic conditions. Although few studies in humans exist at the moment, there is considerable interest from patients and patient groups. The purpose of this review is to examine the existing literature for CAP therapy in inflammatory and infectious dermatologic conditions in humans.
Methodology
A scoping review of the literature was conducted and reported according to PRISMA for Scoping Reviews.17 Relevant literature was identified through structured searches developed for PubMed, Embase (Elsevier), Web of Science (Clarivate), CINAHL (Ebsco). In addition, the first 370 references from search engine Google Scholar were included. Databases were selected as advised by Bramer.18 Search strings were designed with the assistance of the Excel tool developed by Brunskill and final search strings are provided in [appendix 1].19 All dates and languages were included in the search.
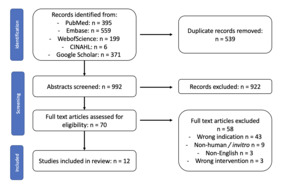
Relevant literature was selected for inclusion if they were English language articles, studies where the intervention was cold atmospheric plasma therapy, conducted with human subjects and that examined either inflammatory or infectious dermatological conditions. Studies were rejected if they were case reports or literature reviews, non-English language articles, studies where the intervention was not CAP, studies conducted on animals or in vitro (ie, human cell lines) or examining non-inflammatory or infectious conditions, such as but not limited to wound care, pigment diseases, or skin cancer. An initial 50 study random screen was conducted by both ACZ and PL to ensure agreement on screening terms. Afterwards a two-step screening process was performed by ACZ reviewing titles/abstract and full text reviews and PL was consulted in cases of uncertainty. Covidence software (Covidence.org; Melbourne, Australia) was used for all screening steps. (See Figure 2)
The data was extracted by ACZ, utilizing Covidence's extraction tool and exported into an Excel file. The data collected during extraction included the year the study was conducted, the authors, the total number of subjects, the disease being studied, the study design, the population, intervention, and outcome. The aim was to identify any study that would satisfy our search criteria, which was largely randomized controlled trials and some non-controlled trials. Given the lack of articles on human subjects and the novelty of CAP in this specific field, we did not conduct a critical appraisal. Results were grouped according to the conditions that were studied, which were either inflammatory or infectious etiology. Within each table results were ordered alphabetically.
Results
Our search concluded with 12 studies, six of which were inflammatory conditions and six of which were infectious conditions.
Inflammatory Dermatoses
For the inflammatory studies, the conditions identified were radiation dermatitis, pruritus, rosacea, atopic dermatitis, and acne vulgaris. Radiation dermatitis was the topic of two of the studies from the same researchers, and for the remainder of topics, there was one study that covered each. The first radiation dermatitis study was a non-controlled trial for patients undergoing whole breast irradiation (WBI). Given that patients received CAP treatment while undergoing the radiation therapy, there was no initial level of documented radiation dermatitis noted.
The first 3 patients received 60 seconds (s) twice a week of treatment, and given that this was well tolerated, the rest of patients 180s three times a week. Patients were similarly able to tolerate this increase, and last 3 patients received a 120s treatment five times a week. Subjects in the study were satisfied with CAP during WBI and close to 30% believed that CAP had reduced radiation dermatitis.20 The follow-up study of this condition by the same researchers in 2024 showed promising results. Similar to the prior study, the baseline level of radiation dermatitis was not assessed, as the treatment was given during the course of radiation. Per the results of the prior study, the treatment time was set at 120s daily during the duration of their radiation treatment.
The study showed a significant improvement in toxicity (p < 0.001), as well as other symptoms like hyperpigmentation (p < 0.001), edema (p = 0.020), or pain, itching, and burning (p < 0.001).21
Other studies showed significant improvement in lesions treated with CAP for both rosacea and atopic dermatitis. In particular, patients with rosacea treated with CAP 90s daily for 6 weeks had significantly lower Dermatology Life Quality Index (DLQI), decreased erythema size (p = 0.041), and when patients used CAP on both sides of the face for an additional 6 weeks, inflammatory lesions decreased on the former control side (p = 0.03) and kept reducing in the CAP treated side (p = 0.04).22 In patients with atopic dermatitis, treated for 5 minutes in each of three sessions, CAP-treated lesions showed a significant improvement in Atopic Dermatitis Antecubital Severity (ADAS) (p < 0.001) Eczema Area and Severity Index (EASI) (p = 0.002), SCORing Atopic Dermatitis (SCORAD) (p = 0.001), and Visual analogue scale (VAS) (p = 0.032). S. aureus was significantly reduced between CAP and control (p = 0.047).23
Finally, CAP also proved to be effective in reducing the number of inflammatory lesions in acne vulgaris in subjects with moderate to severe, bilateral papulopustular acne. Patients in this study were treated for 4-6 weeks, in which they received 8-10 treatments, each of which lasted between 1 to 10 minutes. CAP-treated inflammatory lesions were significantly reduced at last treatment, two-week, and four-week follow-up (p < 0.05). Subjects reported a significant improvement (p < 0.05) in the Global Aesthetics Improvement scale (GAIS) for the treatment group lesions, even though physician-rated severity of acne was not significantly decreased.24
Only one study in this cohort did not show a significant difference. In this study, subjects with pruritus from a variety of etiologies were treated with CAP or argon gas for 2 minutes a day, during a variable period of time per patient which averaged out to be 4.7 treatments per area. At the end of the study, there was no significant reduction in VAS, and both therapies showed a significant reduction of pruritus (p < 0.0001).25 (See Table 1)
Infectious Dermatoses
Studies for infectious conditions showed a wider variability of results. In some studies, CAP did not show significant improvements compared to control. Overall, for patients with condyloma acuminata, the difference in relapse time between plasma and control group was not significant after 24 weeks. However, HIV-positive patients treated with control did have significantly higher relapse rates (p = 0.010).26 In another study looking at Malassezia folliculitis, patients received 3 minute sessions daily for 2 weeks. The efficacy endpoints for oral itraconazole and CAP both decreased significantly, with no significant difference between DLQI and GAIS scores between groups.27 Finally, patients using CAP compared to ivermectin to treat demodex in patients with rosacea had similar results with CAP. However, this study was highly variable, with one patient who received 4 session over 2 weeks, another receiving 10 session over 6 weeks, and the last one receiving 12 sessions over 6 weeks.28
In one study examining molluscum contagiosum and verruca vulgaris, CAP fared worse than the standard of care. Patients received CAP at 4-week intervals for a maximum of three treatments, though the length of treatments was not specified. Here, lesions treated with liquid nitrogen had a slightly significantly higher median degree of lesion resolution (p = 0.028). However, patients were significantly less likely to experience pain and burning as adverse events with CAP (p < 0.001).29
Conversely, patients with herpes zoster treated with CAP did show significantly better results compared to a heated gas control. In this study, patients received between 3-9 treatments which lasted 5 minutes each, Patients noted significant improvements in overall pain (p < 0.01), vesicle healing on days 1 and 2 (p < 0.05 and p < 0.01, respectively). After day 1 there was a significant reduction as well in erythema (p < 0.01).30 Additionally, a non-controlled trial showed that patients were satisfied with CAP in the treatment of onychomycosis. For 6 months, patients received 3 treatments per week, each lasting between 10-20 minutes. After this, more than half the patients reported achieving resolution.31 However, given the lack of control of this study, it is hard to classify the effectiveness of CAP as compared to standard of care antifungal treatment. (See Table 2)
Discussion
Despite relatively few studies with small numbers, CAP appears to hold promise for some, but not all diseases studied. CAP has more impressive results for inflammatory rather than infectious conditions thus far. While not all studies reported safety and adverse events using CAP, those that did rarely reported any significant adverse events, and reported decreased pain and increased tolerability with CAP.
However, it is challenging to compare these studies, given their marked heterogeneity. Only two of the 12 studies compared the same condition, and the remainder dealt with different conditions that also vary in their standard of care treatment. Beyond the variation in the conditions treated, most of the studies did not use the same device settings and many did not even use the same type of CAP device. This variability further calls any firm conclusions into question. Furthermore, there was a variety of study designs: some were RCTs, some were non-controlled trials, one was a non-randomized controlled trial. Within the RCT and the experimental studies with controls, some had different lesions in the same individual as control, while others designed the control group to be different individuals.
Finally, the duration of the studies also varied significantly, ranging from days to up to 6 months, this also meant that the number of total treatments as well as the frequency at which the treatments were administered also differed immensely. As illustrated by some studies, the effects of CAP only became significant after a certain number of sessions. For example in Karrer et al, there was only a significant decrease in the number of lesions for CAP with 10 treatment sessions and this difference was not appreciated in the lesions that received 8 treatment sessions.24 It is then entirely plausible that subjects in other studies that didn't have the same CAP benefits might not have received sufficient sessions.
While CAP remains an intriguing modality that has been investigated in several conditions, further research is needed, with larger, more rigorous trial design and replication of results.
Disclosures
ACZ has no conflicts of interest to declare.
TG has no conflicts of interest to declare.
Dr. Lio reports research grants/funding from AbbVie, AOBiome; is on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oréal, MyOR Diagnostics, ParentMD, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, Verrica; reports consulting/advisory boards for Alphyn, AbbVie, Almirall, Amyris, Arcutis, ASLAN, Boston Skin Science, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs, Concerto Biosci, Dermavant, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Kimberly-Clark, LEO Pharma, Lipidor, L’Oréal, Merck, Micreos, MyOR Diagnostics, Regeneron/Sanofi Genzyme, Skinfix, Theraplex, UCB, Unilever, Verrica Yobee Care; stock options with Codex Concerto Biosciences and Yobee Care. In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member of the National Eczema Association.
We confirm that this manuscript is original, has not been published previously or under consideration by another journal, does not infringe upon any copyright of a third party, and will not be published elsewhere whether online or in print once accepted.
This research received no funding.
_on_skin.png)
_on_skin.png)